For Scientists
The goal of the Accelerating Medicines Partnership (AMP®) SCZ program is to build tools to improve success in developing pharmacologic treatments for patients with clinical high risk (CHR) for psychosis. The initiative also seeks to develop and validate biomarkers and outcome measures that can establish early indicators of pharmacologic treatment efficacy. We will recruit a cohort of participants at CHR for psychosis and use repeated measurements of multi-modal biomarkers, endpoint measures, and clinical outcomes to accomplish these goals.
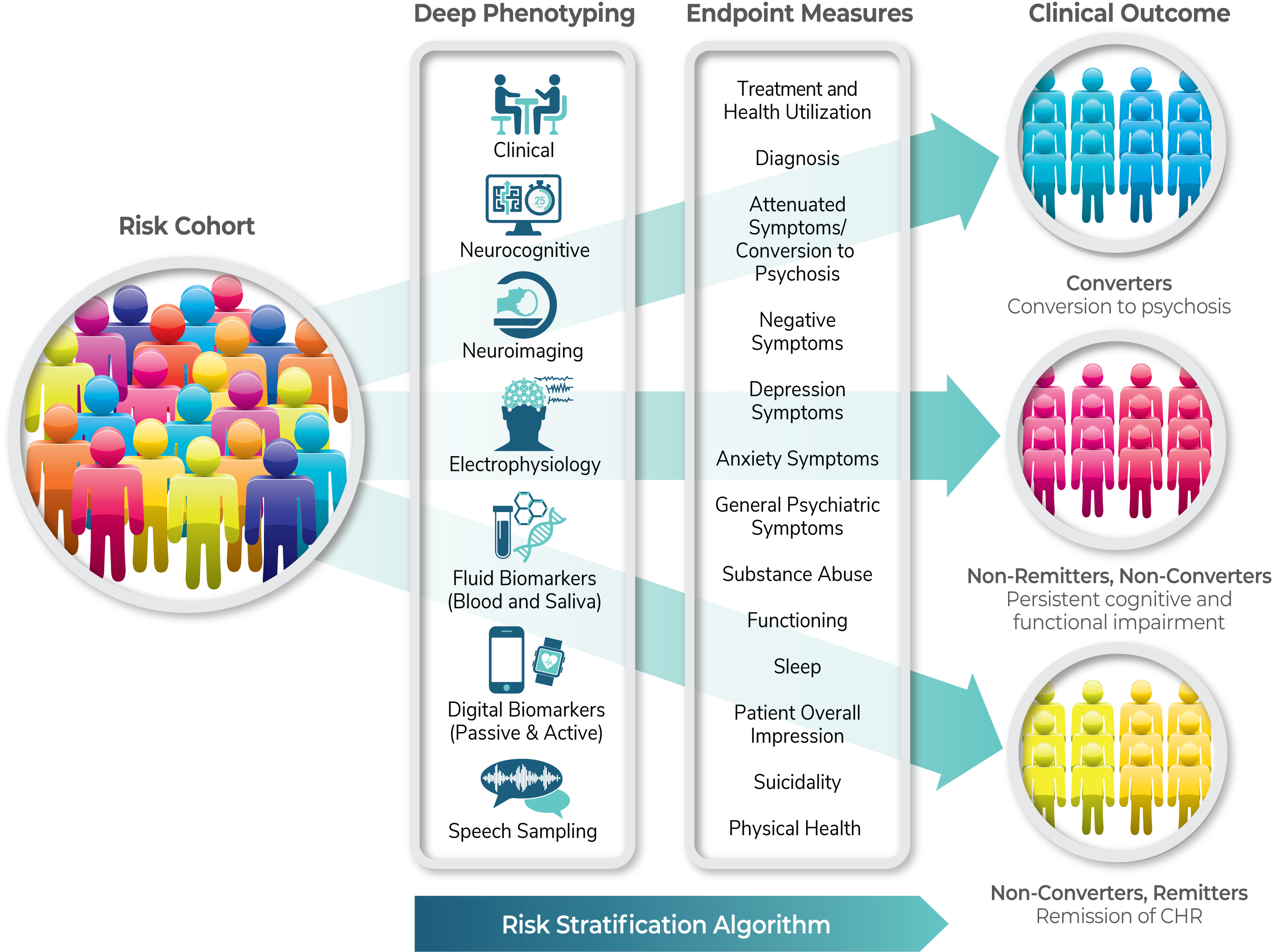
The graphic below illustrates the clinical (including demographic and socio-economic measures) and multi-modal brain, digital, and fluid-based biomarker approach for deeply phenotyping the CHR cohort. The endpoint measures are aligned with the three clinical outcomes.
In this section of the website, scientists can learn more about the AMP SCZ program study design, protocols, standard operating procedures (SOPs), data sharing, and software tools.
Last Reviewed on May 2, 2022
